Ring Recalls Their Video Doorbells Due to Potential Fire Risk! (Is Yours Affected?)
I hope everyone is listening up! Ring has issued a recall on their video doorbells due to fire hazard! This affects nearly 350,000 of their products for both the stain nickel and bronze editions. This recall involves Ring Video Doorbell (2nd Generation), model number 5UM5E5 smart doorbell cameras. The video doorbells have a blue ring at the front. They were sold with a mounting bracket and a USB charging cable. The two-way audio doorbell can be hardwired or battery-powered and supports night vision. The Ring logo is printed on the bottom front of the doorbell and the model and S/N are on a label on the back of the doorbell and the outer packaging. You can determine if your doorbell is included in this recall by entering the doorbell’s serial number at http://support.ring.com/ring-2nd-gen-recall. Only Ring Video Doorbell (2nd Generation) models with certain serial numbers are included.
FDA Recalls Hand Sanitizers Due to Risk of Methanol Contamination!
FDA is warning consumers and health care providers that the agency has seen a sharp increase in hand sanitizer products that are labeled to contain ethanol (also known as ethyl alcohol) but that have tested positive for methanol contamination. Methanol, or wood alcohol, is a substance that can be toxic when absorbed through the skin or ingested and can be life-threatening when ingested.
Here are the 3 that have been recalled but you can find a complete list of recalled hand sanitizer on the FDA web site, here.
- Saniderm Advanced Hand Sanitizer (1L) Bottles
- Bottles will have lot number 53131626 and are manufactured on April 1, 2020. (These were sold in Virginia, Maryland and New Jersey)
- Bottles will have lot number 0530 and expiration date of 4/2022. (These were sold nationwide)
- All Clean Hand Sanitizer, Moisturizer and Disinfectant (1L) Bottles
- Bottles will have UPC code 628055370130 (These were sold nationwide)
- Mystic Shield Protection Topical Solution (8.45oz) Bottles
- Bottles were distributed between May 21 – June 30, 2020 and will have a blue or green label with white or transparent cap. (Sold in California, Louisiana, Massachusetts and Texas)
Blumen Hand Sanitizer Recall! Did you get this Sam’s Club deal?
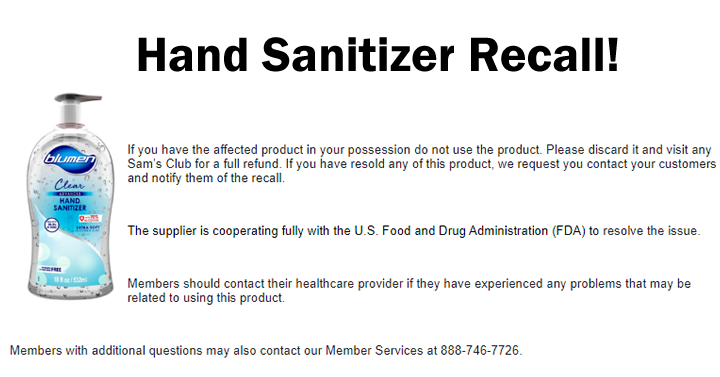 The U.S. Food and Drug Administration (FDA) is warning consumers not to use hand sanitizers from 4 E Global SAPI de CV (Mexico) or with the Blumen product name. These products are labeled to contain ethanol (also known as ethyl alcohol) but have tested positive for methanol contamination. Methanol, or wood alcohol, is a substance that can be toxic and life-threatening when absorbed through the skin or ingested. This product is Blumen Clear Advanced Hand Sanitizer (18 oz.) with the UPC 81426602391 4.
The U.S. Food and Drug Administration (FDA) is warning consumers not to use hand sanitizers from 4 E Global SAPI de CV (Mexico) or with the Blumen product name. These products are labeled to contain ethanol (also known as ethyl alcohol) but have tested positive for methanol contamination. Methanol, or wood alcohol, is a substance that can be toxic and life-threatening when absorbed through the skin or ingested. This product is Blumen Clear Advanced Hand Sanitizer (18 oz.) with the UPC 81426602391 4.
If you have the affected product in your possession do not use the product. Please discard it and visit any Sam’s Club for a full refund. If you have resold any of this product, we request you contact your customers and notify them of the recall. Members should contact their healthcare provider if they have experienced any problems that may be related to using this product. Members with additional questions may also contact our Member Services at 888-746-7726.
For more details on the FDA warning see their website here.


